- FHAIVE is a research institute devoted to the development and validation of integrated approaches (IATA).
- IATA allow new knowledge to be gathered, concerning the chemical-biological systems interactions.
- In FHAIVE, IATA are developed by integrating advanced in vitro models with toxicogenomics and AI-enabled advanced data modelling.
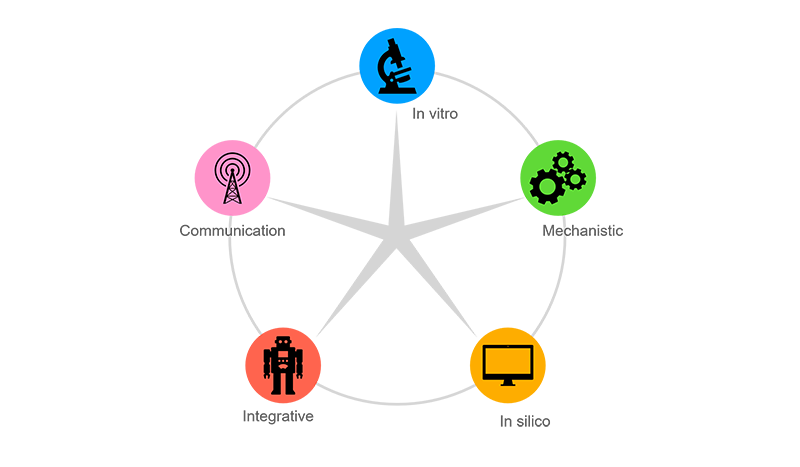
- At FHAIVE operates in five areas of interest: in vitro, mechanistic, in silico, integrative, and communication.
- FHAIVE is part of the Faculty of Medicine and Health Technology (MET), Tampere University, Finland